1/8ページ
ダウンロード(543.3Kb)
Enhancing efficiency and economics in process development and manufacturing of biotherapeutics
ホワイトペーパー
このカタログについて
| ドキュメント名 | Enhancing efficiency and economics in process development and manufacturing of biotherapeutics |
|---|---|
| ドキュメント種別 | ホワイトペーパー |
| ファイルサイズ | 543.3Kb |
| 取り扱い企業 | ザルトリウス・ジャパン株式会社 (この企業の取り扱いカタログ一覧) |
この企業の関連カタログ

このカタログの内容
Page1
APPLICATION NOTE 11
Enhancing efficiency and economics in process
development and manufacturing of biotherapeutics
Rashi Takkar, Applications Scientist; Sriram Kumaraswamy, Director, Marketing Field Applications
Introduction to improve the efficiency and economics in all stages of devel-
opment. These key drivers have fueled the search for innovative
Analytical techniques that measure protein quantity and quality analytical techniques that provide improved performance and
are used in nearly all stages of research, process development speed without increasing costs. Biopharmaceutical companies
and manufacturing of biotherapeutics. UV spectroscopy, ELISA have enthusiastically adopted ForteBio’s Octet® systems due to
and HPLC have been in use for decades for protein quantitation their broad utility in protein quantitation and functional character-
in physiological and process samples, and continue to be the ization combined with enhanced throughput, decreased sample
workhorses despite their many limitations. To characterize the preparation requirements, and low cost of operation. This white
functional activity of proteins during biotherapeutic development, paper describes the use of Octet instruments for protein quan-
label-free biosensor-based binding assays are increasingly being titation, particularly in the areas of process development and
utilized. The high cost and lengthy times associated with drug quality control.
discovery and development have forced biopharm companies
Target ID & Lead screening Lead optimization Preclinical Process QC &
validation & selection & characterization development development manufacturing
Mechanism Screening Anity Pharmacokinetics Cell line develop.–
of action – for binders maturation – (PK) titer and growth Activity assay
biomolecular (hybridoma, phage binding kinetics media assessment
interactions or lysates)
Pharmacodynamics FcRn binding
Fc engineering/ (PD) Chromatography assay
ELISA assay Anity/ on-rate/ humanization – conditions
development o-rate ranking FcRn binding optimize – DBC,
of clones binding, wash,
Immunogenicity Binding
elution and CIP kinetics
Epitope mapping
Epitope binning Contaminant
testing – insulin,
HCP and residual
Mammalian Protein A
clone selection
for scale-up
Loading
concentration for
Quantitation small-scale
specific purification
Figure 1: Applications of Octet instruments in the drug research and development process.
1
Page2
Protein quantitation of 70 complex samples
Octet systems
Sample and
instrument prep Sample analysis
30 min 10 min
60 min total assay time
40 min operator hands-on time
Sample run time
20 min
HPLC
Sample and
instrument prep Sample injection and run time Sample analysis
4 hr 18 hr 50 min
23 hr total assay time
5 hr operator hands-on time
ELISA
Bind capture Sample and
antigen to plate instrument prep Sample run time Sample analysis
Overnight 1 hr 4 hr 30 min
21.5 hr total assay time
5.5 hr operator hands-on time
Figure 2: Comparison of protein quantitation in complex matrices using Octet systems and alternative methods.
Advantages over ELISA and HPLC analyte concentration in crude matrices such as cell culture
supernatant, cell lysate and serum. Octet concentration assays
The principles of concentration measurement with an Octet
system are similar to established immunoassays such as ELISA are complemented by the platform’s ability to measure func-
and HPLC. However, protein quantitation protocols on the tional activity. For example, titer for a monoclonal antibody
Octet platform provide several advantages. The Octet plat- (mAb) can be determined using biosensors coated with Protein
form monitors binding of proteins from solution to a biosensor A, while the functional activity of the mAb can be assessed in
surface in real time, without need for labels or other detection a second assay step involving binding to its specific antigen.
reagents. This real-time monitoring of binding interactions In contrast, HPLC and A280 spectroscopy can determine
enables clear discrimination between specific and non-specific only the total protein concentration of a sample, and separate
binding signals, which can shorten assay development times assays must be used to measure biological activity.
dramatically. Octet assays are also much faster: quantitation
of a 96-well plate requires 15–30 minutes, or 60 minutes for
a 384-well plate, depending on the instrument model. Fig- Bio-Layer Interferometry technology (BLI)
ure 2 provides a comparison of analysis times. Analysis of 70 BLI technology monitors and analyzes the interference pattern
samples on an Octet RED96 system requires as little as 55 generated from the reflection of white light from two different
minutes including operator hands-on time, whereas ELISA or surfaces: a layer of immobilized protein on the biosensor tip and
HPLC assays require at least 22 hours including several hours an internal reference layer (Figure 3). Any increase or decrease
of analyst involvement. Samples run on Octet systems are also in the number of binding molecules to the biosensor surface
recoverable, so that researchers may save and reuse precious produces a change in optical thickness that causes a shift in the
sample for other experiments. In addition, Octet assays are not
affected by absorption interferences in colored samples or by interference pattern. Unbound molecules in complex matrices
light scattering with turbid samples, enabling measurement of and changes in the refractive index of the surrounding medium
have minimal effect on the interference pattern. BLI technology
2
Page3
1.0
Incident BLI signal
white processing 0.8
light
0.6
Biocompatible
surface 0.4
Bound
molecule 0.2
Unbound molecules
have no effect
Wavelength (nm)
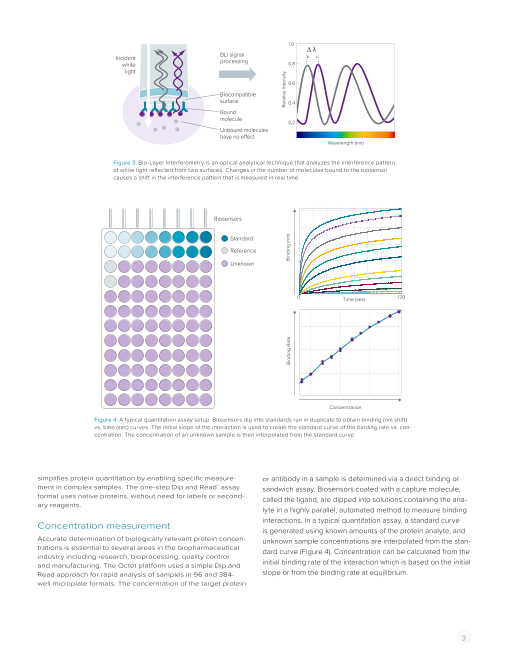
Figure 3: Bio-Layer Interferometry is an optical analytical technique that analyzes the interference pattern
of white light reflected from two surfaces. Changes in the number of molecules bound to the biosensor
causes a shift in the interference pattern that is measured in real time.
Biosensors
Standard
Reference
Unknown
0 Time (sec) 120
Concentration
Figure 4: A typical quantitation assay setup. Biosensors dip into standards run in duplicate to obtain binding (nm shift)
vs. time (sec) curves. The initial slope of the interaction is used to create the standard curve of the binding rate vs. con-
centration. The concentration of an unknown sample is then interpolated from the standard curve.
simplifies protein quantitation by enabling specific measure- or antibody in a sample is determined via a direct binding or
ment in complex samples. The one-step Dip and Read™ assay sandwich assay. Biosensors coated with a capture molecule,
format uses native proteins, without need for labels or second- called the ligand, are dipped into solutions containing the ana-
ary reagents.
lyte in a highly parallel, automated method to measure binding
Concentration measurement interactions. In a typical quantitation assay, a standard curve
is generated using known amounts of the protein analyte, and
Accurate determination of biologically relevant protein concen- unknown sample concentrations are interpolated from the stan-
trations is essential to several areas in the biopharmaceutical dard curve (Figure 4). Concentration can be calculated from the
industry including research, bioprocessing, quality control
and manufacturing. The Octet platform uses a simple Dip and initial binding rate of the interaction which is based on the initial
Read approach for rapid analysis of samples in 96 and 384- slope or from the binding rate at equilibrium.
well microplate formats. The concentration of the target protein
3
Relative Intensity
Binding Rate Binding (nm)
Page4
Quantitation applications for drug Titer assessment and growth media optimization
development using the Octet platform
RESEARCH AND EARLY BIOPROCESS
DEVELOPMENT
The Octet platform is a useful tool for cost-effective protein
expression screening in research and early bioprocess devel- Pe-adapted
opment with several significant benefits. Host Cells Vector
Octet platform advantages
Transfections
• Antibody and protein concentrationst can be determined in
crude matrices, such as cell lysates or hybridoma superna- 96-well Plates
tants, saving time and resources when processing a large Range: 1–300 µg/mL
Throughput: 1000s clones
number of samples.
• Octet assays have a dynamic range of greater than two or-
Octet system
ders of magnitude, enabling a single quantitation assay to be 24-, 12- and 6-well Plates
utilized across all development stages – from early cell culture Range: 1–500 µg/mL
Throughput: 200–500 clones
to production bioreactors.
• Octet systems perform rapid quantitation with minimal user in- Octet system
volvement. 96 samples are analyzed in as little as 20 minutes,
and 384 samples in 70 minutes. With additional plate handling T-flasks
automation, Octet 384 systems can process more than 1200 Range: 1–500 µg/mL
samples per day. Throughput: 100–150 clones
• Samples are analyzed in a non-destructive method and are
fully recoverable, which is advantageous when working with
low sample volumes and precious samples. Octet system
• Octet systems are easy to learn and operate. Multiple analysts
can operate the instrument with minimal training, allowing Shake Flasks
rapid integration of these systems into laboratory workflows. Range: 1–700 µg/mL
Throughput: 50–100 clones
Early clone selection
In clone selection, thousands of hybridoma or phage clones
are screened to determine positive binding clones and their Octet system
protein secretion levels. Titer measurements are used to select
high-producing clones and to normalize the functional activ- Fed-batch Shake Flasks
ity of these clones in crude matrices. Integration of an Octet Range: 1–700 µg/mL
system into the antibody discovery workflow affords increased Throughput: 12–20 clones
screening throughput. With Octet RED384 and Octet QK384
instruments, automated plate handling can also be added to
achieve even higher throughput. Octet quantitation assays Octet system
are also used to determine loading levels of chromatography
columns for small-scale purification.
Cell line development 1–3 L Seed Bioreactors
Harvest samples are screened on Octet systems to select Range: 1–4 g/L
Throughput: 2–5 clones
high-expressing clones during various scale-up procedures
involving 96-, 48-, 24- and 6-well plates, fed batch shake flasks,
and bioreactors (Figure 5). Octet assays also are used to de-
termine protein levels during media development for fed-batch
and bioreactor processes (Figure 5). This is performed by com- Figure 5: Protein titer assessment and growth media optimization using
paring protein secretion levels following variations in feeding the Octet system at different stages of cell line development.
regimes, strategies and concentrations. Data acquisition and
4
Page5
Polishing
Cell culture Anity chromatography Viral Ultrafiltration
harvest chromatography (at least 2 steps) filtration diafiltration
Optimizing dynamic binding Optimizing dynamic binding Optimize different
capacity of column resins capacity of resins formulations based on:
• Detect protein breakthrough • Bind and elute mode: determine • Protein concentration
in flow-through in crude protein breakthrough point required for stability
matrix • Flow-through mode: determine • Protein activity/binding
Optimizing binding, wash and final fraction when target protein
elution conditions detectable
• Protein binding/activity Optimizing binding, elution, and
• Residual Protein A wash conditions
• HCP (general & specific) • Protein recovery
• Protein recovery • Protein binding/activity
• Residual protein A
• HCP
Figure 6: Use of Octet systems in the downstream purification process of proteins and antibodies.
subsequent data analysis can be performed rapidly for hun- containing a known concentration of target protein and moni-
dreds of samples, bypassing traditional processing bottlenecks. toring this protein in the flow-through fractions. Quick determi-
Please see ForteBio Application Note 13, Fc-Fusion Protein nation of DBC using HPLC or A280 spectroscopy is hampered
Quantitation in Cell Culture Supernatants, for more information. by the presence of large amounts of host cell proteins in the
flow-through fractions. Specific detection of the protein of inter-
DOWNSTREAM PROCESS DEVELOPMENT est among contaminants is straightforward with Octet systems,
reducing the time required to optimize purification conditions
Efficient development of manufacturing processes for anti- (Figure 6).
bodies and recombinant proteins is a critical need for bio-
pharmaceutical companies. Increasingly stringent regulatory
requirements targeting better understanding and control of Binding, wash and elution conditions
manufacturing processes are expected to impact product qual- Numerous chromatography binding and elution conditions are
ity and performance. The Octet platform can quickly determine tested during optimization studies, including different buffer
the impact of multiple process variables at different stages of compositions, salt, pH, operating temperature and sample
the purification process, and help identify optimal conditions injection volume. High-throughput tools, such as mini columns
that provide protein product with the desired yield, binding and 96-well filter plates, often are used to screen these process
specificity and potency (Figure 6). Pre-configured reagents variables. The impact of different conditions on product titer
and protocols are available for rapid quantitation of protein and quality can be analyzed rapidly and effectively on Octet
products, host cell proteins (HCP), and residual Protein A levels systems, speeding identification of optimal chromatography
during purification processes. conditions (Figure 6).
Octet platform advantages Contaminant testing
• One Octet instrument can be used to measure protein titer, Downstream purification processes must remove host cell pro-
host cell proteins and residual Protein A contaminant levels. teins, residual Protein A and residual DNA impurities. According
• Octet assays are faster to develop and run than ELISA and to guidance from regulatory authorities, host cell proteins in a
HPLC assays. drug substance should be below detectable levels using a high-
ly sensitive analytical method, and as a rule this level should not
• Octet assays can be automated with robotic and liquid han- exceed 100 ppm. The type of assay required for HCP determi-
dling systems for complete, walk-away screening. nations depends on the phase of clinical studies for which the
material is produced. For earlier clinical phases, a generic assay
Dynamic binding capacity (DBC) of chromatography columns may be sufficient. However, a process-specific HCP assay gen-
Affinity chromatography often is the first major purification pro- erally is required for phase 3 and later studies. Leached Protein
cedure performed on harvested cell culture samples in down- A is another contaminant of concern in process development.
stream bioprocessing. The dynamic binding capacity (DBC) of The elution of antibodies during Protein A chromatography
an affinity chromatography column is defined as the amount requires acidic conditions, which in turn can accelerate leaching
of protein that will bind to the column resin under a defined of Protein A from the column. Residual Protein A levels should
condition. DBC is determined by continuously loading a sample not exceed 10 ppm in the final drug product.
5
Page6
Customer highlight: GlaxoSmithKline Benefits of automated Octet CHO HCP assay compared to
manual ELISA
The analytical lab at GlaxoSmithKline incorporated a generic
HCP assay on the Octet QK384 system to streamline their Benefit Details
workflow in process development. The automated Octet HCP
Precision Liquid handling robot reduces pipetting variation
assay required minimal analyst intervention and provided inherent in manual pipetting
more accurate and precise results than their manual ELISA
assay (Figure 7). Hands-on time for preparation and process- Reliability Method performed exactly the same each time
ing of 1–3 assay plates was reduced to 30 minutes from the Streamlined Worklist drives robotic method and creates sample
previous 2.5 hours with manual ELISA, and antibody consump- process plate importation files
Robotic method automatically creates and executes
tion decreased by 40%. Octet method file
More information on the development of the HCP assay on Walk away No analyst intervention needed to complete method
Octet systems can be found in Technical Note 24, Host Cell after instrument loaded and diluent volumes are
checked
Protein Dectection on the ForteBio website.
Washing No washing steps needed and plate washer
Process development assays for residual Protein A and prod- steps integration not required
uct titer can be fully automated on Octet 384 systems using Analysts Automated Octet ~30 minutes for 1–3 assay plates
external liquid handling platforms. The Octet assay for leached involvement Manual ELISA ~2.5 hours per assay plate
Protein A is highly sensitive with a LLOQ of 0.20 ppm, has >2.5
Throughput 3 assay plates can be run in ~5 hours
logs of dynamic range, and is faster than competing methods. 38 samples/plate in duplicate wells > 108 samples in
A residual Protein A assay on the Octet RED384 system can 3 plates
be completed in 1 hour and 45 minutes per plate with minimal Antibody Re-use of coating antibody can significantly reduce
analyst involvement, compared to a minimum of 3.5 hours for consumed consumption over multiple assay plates
ELISA (including significant analyst hands-on time). For more
information on the Octet residual Protein A quantitation proto- Figure 7: Benefits of automated Octet CHO HCP assay compared to manu-
al ELISA summarized by GSK.
col, see Technical Note 18, Dip and Read Residual Protein A
Detection Kit on the ForteBio website.
QUALITY CONTROL
Octet platform advantages
Octet systems provide robust and highly reproducible assays • Octet systems are designed for GLP/GMP environments, and
for protein concentration and functional activity, and are suit-
provide 21 CFR Part 11 compliance tools.
able for operation in quality control and manufacturing environ-
ments. Protein activity and various kinetic assays are used to • Octet assays provide detailed information about the binding
support in-process testing, drug potency, lot-to-lot variability behavior of protein products, and reveal subtle differences in
and stability studies. binding activity between production lots.
Activity assay Quantitation assay
Streptavidin
Biotin Fab
Ligand — binds Fab Antibody Fc Protein A — binds Fc
Figure 8: An activity assay can be developed on the Octet platform by immobilizing a specific biotinylated ligand on the biosensor and then detecting binding
of an analyte, FAb or protein. In the quantitation assay, mAb titer is determined using Protein A-loaded biosensors, which does not measure mAb activity
towards its target.
6
Page7
Customer highlight: Aragen Biosciences • Octet quantitation assays provide a direct measure of the
biological activity of the analyte(s) (Figure 8).
Aragen Biosciences created a stable and scalable CHO cell
line, purification platform and manufacturing process for a • Octet assays can be easily transferred to manufacturing oper-
particular product in a GMP environment. They developed ations.
an Octet assay to compare the activity and quality of a new
product lot (Lot 2) with a reference lot (Lot 1) throughout Activity assays
their bioprocess and manufacturing processes. The as- An activity assay is generally utilized during process develop-
say involved loading a biotinylated ligand on Streptavidin ment, QC and manufacturing to compare various prepared lots
biosensors, and measuring binding interaction of the ligand of the drug molecule, as well as its stability. Activity assays are
with the protein analyte. As seen in Figure 9, Lot 2 contained critical because they differentiate active protein from inactive
a large second peak that was absent in the Lot 1 reference or clipped variants, as those species will not bind the ligand.
material. The second peak in Lot 2 exhibited a slower on-rate Active protein concentration can be determined using a binding
and much faster off-rate, indicative of a less-active fraction assay on the Octet platform by immobilizing a specific ligand
(Figure 10). Octet system activity data results were confirmed against the target analyte onto the biosensor, and then measur-
with a cell-based assay, and Aragen was able to modify their ing its binding interaction with the analyte as shown in Figure 8.
production conditions to significantly reduce this second
peak fraction.
Lot 1 Lot 2
High Specific Low Specific
Binding Activity Binding Activity
Peak 2
Presence of large
Peak 1 second peak
Peak 1 correlated with
reduced specific
Peak 2 binding activity
30 35 30 35
Figure 9: HPLC spectra of Lot 1 and Lot 2 of a drug molecule. Lot 2 was made by Aragen Biosciences and had
an additional peak (Peak 2) compared to the reference lot (Lot 1) provided by their customer. Data provided
courtesy of Aragen Biosciences.
Peak 2
Peak 1
30 35
A lower on-rate and an
increased o -rate was
clearly indicative of a
less-active fraction
Figure 10: The Octet binding kinetics or functional assay demonstrated that Peak 1 was the active fraction. Peak 2 was the less-active fraction,
with a lower on-rate and a much faster off-rate in a binding experiment. Data provided courtesy of Aragen Biosciences.
7
Page8
Conclusion
Octet systems deliver comprehensive characterization of the high throughput needed to screen through large libraries of
biotherapeutics, as well as rapid and reproducible determi- candidate drug molecules. In later stages of process develop-
nation of protein concentrations during different stages of the ment and manufacturing, Octet systems provide the required
development process. Titer and functional activity assays on reliability, robustness and measurement accuracy. The broad
Octet systems are useful for a broad array of applications in utility of this single platform makes the Octet instrument unique
target identification, lead selection, process development, in its ability to deliver high value across a wide range of appli-
formulation development, quality control, and manufacturing. cation needs in biopharmaceutical discovery, development and
In early stages of drug development, Octet systems provide manufacturing processes.
ForteBio ForteBio Analytics (Shanghai) Co., Ltd. Molecular Devices (UK) Ltd. Molecular Devices (Germany) GmbH
47661 Fremont Boulevard No. 88 Shang Ke Road 660-665 Eskdale Bismarckring 39
Fremont, CA 94538 Zhangjiang Hi-tech Park Winnersh Triangle 88400 Biberach an der Riss
888.OCTET-75 or 650.322.1360 Shanghai, China 201210 Wokingham, Berkshire Germany
www.fortebio.com fortebio.info@moldev.com salesops.china@moldev.com RG41 5TS, United Kingdom + 00800 665 32860
+44 118 944 8000
uk@moldev.com
©2019 Molecular Devices, LLC. All trademarks used herein are the property of Molecular Devices, LLC. Specifications subject to change without
notice. Patents: www.moleculardevices.com/product patents. FOR RESEARCH USE ONLY. NOT FOR USE IN DIAGNOSTIC PROCEDURES.
AN-4011 Rev B