1/13ページ
ダウンロード(2.8Mb)
Application Note 19「Analysis of FcRn-Antibody Interactions on the Octet platform」
ホワイトペーパー
このカタログについて
| ドキュメント名 | Application Note 19「Analysis of FcRn-Antibody Interactions on the Octet platform」 |
|---|---|
| ドキュメント種別 | ホワイトペーパー |
| ファイルサイズ | 2.8Mb |
| 取り扱い企業 | ザルトリウス・ジャパン株式会社 (この企業の取り扱いカタログ一覧) |
この企業の関連カタログ

このカタログの内容
Page1
APPLICATION NOTE 19
Analysis of FcRn-antibody interactions on the Octet
platform
Renee Tobias and Weilei Ma, ForteBio
Introduction The Octet platform for analyzing FcRn-
The Fc region of human IgG contributes to a number of benefi- antibody interactions
cial biological and pharmacological characteristics of therapeu- The Octet family of instruments is based on Bio-Layer Interfer-
tic antibodies. One of the most important is prolonging plasma ometry (BLI), a label-free biosensor technology that measures
half-life, due to its unique, pH-dependent interaction with the molecular interactions in real time for the purpose of detection,
neonatal Fc-receptor (FcRn). Because altered FcRn binding can quantitation and kinetic analysis (Figure 1). Octet instruments
increase or decrease serum half-life of Fc-containing therapeu- can read from 2 to 96 samples simultaneously in automated for-
tics, thereby impacting drug efficacy, FcRn binding interactions mat using a standard microplate for rapid determination of bind-
are increasingly being assessed at multiple stages of biologic ing affinity constants (KD), association rates (ka or on-rate) and
drug development. FcRn-Fc activity and binding assays are dissociation rates (kd or off-rate). In addition to complete kinetic
performed as part of characterization studies to enhance over- characterization, equilibrium assays can be used to determine
all product understanding and demonstrate comparability in the KD, or simple binding assays performed to rapidly evaluate rela-
development of biosimilars. Commonly used in vitro methods tive affinity or screen large numbers of samples. BLI biosensors
for analysis of FcRn binding include ELISA, SPR, and bead- are compatible with a wide range of sample types and buffers,
based proximity assays. including cell extracts, serum, and media.
The Octet® system from ForteBio, in conjunction with dis- The standard microplate format combined with disposable Dip
posable Dip and Read™ biosensors, offers users the ability and Read biosensor technology enables automated, highly
to accurately assess FcRn - IgG binding in high throughput, parallel processing on the Octet system in sample volumes as
versatile, and easy to use format. In this application note, assay low as 40 µL. Hands-on time is significantly reduced on the
design and best practices for FcRn-IgG kinetic analysis on the Octet system when compared with the multiple incubation and
Octet system will be discussed, as well as considerations for wash steps required for ELISA or complex assay development
assay optimization, data acquisition, curve fitting, and analysis required for bead-based assays. A wide array of biosensor
of results. specificities enables flexibility in assay formatting. In contrast
1.0
Incident BLI signal
white processing 0.8
light
0.6
Biocompatible
surface 0.4
Bound
molecule 0.2
Unbound molecules
have no effect
Wavelength (nm)
Figure 1: BLI measurement using Dip and Read biosensors. BLI is an optical analytical technique that analyzes the inter-
ference pattern of white light reflected from two surfaces. Changes in the number of molecules bound to the biosensor tip
causes a shift in the interference pattern that is measured in real time.
1
Relative Intensity
Page2
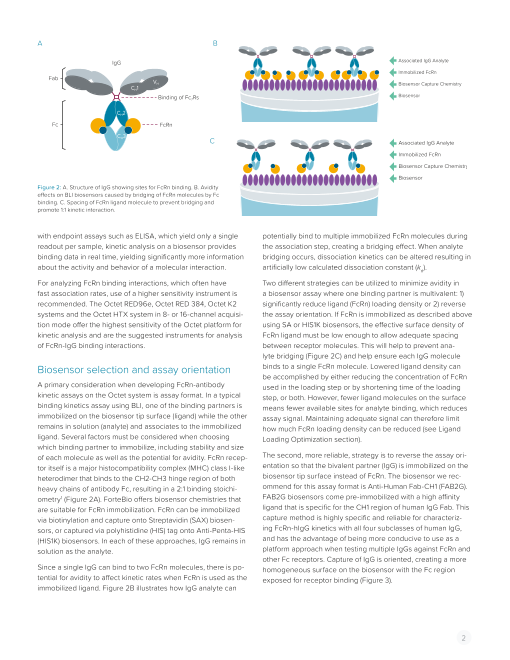
A B
IgG Associated IgG Analyte
Immobilized FcRn
Fab VH Biosensor Capture Chemistry
CH1
Binding of Fc7Rs Biosensor
CH2
Fc FcRn
CH3
C Associated IgG Analyte
Immobilized FcRn
Biosensor Capture Chemistry
Biosensor
Figure 2: A. Structure of IgG showing sites for FcRn binding. B. Avidity
effects on BLI biosensors caused by bridging of FcRn molecules by Fc
binding. C. Spacing of FcRn ligand molecule to prevent bridging and
promote 1:1 kinetic interaction.
with endpoint assays such as ELISA, which yield only a single potentially bind to multiple immobilized FcRn molecules during
readout per sample, kinetic analysis on a biosensor provides the association step, creating a bridging effect. When analyte
binding data in real time, yielding significantly more information bridging occurs, dissociation kinetics can be altered resulting in
about the activity and behavior of a molecular interaction. artificially low calculated dissociation constant (kd).
For analyzing FcRn binding interactions, which often have Two different strategies can be utilized to minimize avidity in
fast association rates, use of a higher sensitivity instrument is a biosensor assay where one binding partner is multivalent: 1)
recommended. The Octet RED96e, Octet RED 384, Octet K2 significantly reduce ligand (FcRn) loading density or 2) reverse
systems and the Octet HTX system in 8- or 16-channel acquisi- the assay orientation. If FcRn is immobilized as described above
tion mode offer the highest sensitivity of the Octet platform for using SA or HIS1K biosensors, the effective surface density of
kinetic analysis and are the suggested instruments for analysis FcRn ligand must be low enough to allow adequate spacing
of FcRn-IgG binding interactions. between receptor molecules. This will help to prevent ana-
lyte bridging (Figure 2C) and help ensure each IgG molecule
Biosensor selection and assay orientation binds to a single FcRn molecule. Lowered ligand density can
be accomplished by either reducing the concentration of FcRn
A primary consideration when developing FcRn-antibody used in the loading step or by shortening time of the loading
kinetic assays on the Octet system is assay format. In a typical step, or both. However, fewer ligand molecules on the surface
binding kinetics assay using BLI, one of the binding partners is means fewer available sites for analyte binding, which reduces
immobilized on the biosensor tip surface (ligand) while the other assay signal. Maintaining adequate signal can therefore limit
remains in solution (analyte) and associates to the immobilized how much FcRn loading density can be reduced (see Ligand
ligand. Several factors must be considered when choosing Loading Optimization section).
which binding partner to immobilize, including stability and size
of each molecule as well as the potential for avidity. FcRn recep- The second, more reliable, strategy is to reverse the assay ori-
tor itself is a major histocompatibility complex (MHC) class I-like entation so that the bivalent partner (IgG) is immobilized on the
heterodimer that binds to the CH2-CH3 hinge region of both biosensor tip surface instead of FcRn. The biosensor we rec-
heavy chains of antibody Fc, resulting in a 2:1 binding stoichi- ommend for this assay format is Anti-Human Fab-CH1 (FAB2G).
ometry1 (Figure 2A). ForteBio offers biosensor chemistries that FAB2G biosensors come pre-immobilized with a high affinity
are suitable for FcRn immobilization. FcRn can be immobilized ligand that is specific for the CH1 region of human IgG Fab. This
via biotinylation and capture onto Streptavidin (SAX) biosen- capture method is highly specific and reliable for characteriz-
sors, or captured via polyhistidine (HIS) tag onto Anti-Penta-HIS ing FcRn-hIgG kinetics with all four subclasses of human IgG,
(HIS1K) biosensors. In each of these approaches, IgG remains in and has the advantage of being more conducive to use as a
solution as the analyte. platform approach when testing multiple IgGs against FcRn and
other Fc receptors. Capture of IgG is oriented, creating a more
Since a single IgG can bind to two FcRn molecules, there is po- homogeneous surface on the biosensor with the Fc region
tential for avidity to affect kinetic rates when FcRn is used as the exposed for receptor binding (Figure 3).
immobilized ligand. Figure 2B illustrates how IgG analyte can
2
Page3
Equilibration Loading Baseline Association Dissociation
FAB2G Biosensor: IgG FcRn
Factory-immobilized
anti-human Fab-CH1 ligand
Figure 3: Workflow for FcRn-hIgG kinetic assay on Anti-Human FAB-CH1 (FAB2G) biosensors.
Assay optimization FcRn. The ligand loading step can be run in ForteBio’s 1x Kinetics
buffer (which contains BSA) for best immobilization results. When
As with any kinetic assay, it is critical to perform proper assay
changing buffers between assays steps - i.e. changing from 1x
development when analyzing FcRn binding interactions so that
kinetics buffer in the loading step to pH 6 assay buffer in baseline
resulting affinity and kinetic constants will be accurate, reliable
and association step — be sure to run the baseline for enough
and reproducible. Quality of the kinetic data depends on using
time to allow biosensors to equilibrate in the new buffer and for
optimal conditions for the biosensor format and the binding pair.
any signal drift to stabilize. A one to five minute baseline step is
Consideration must be given to assay components such as li-
usually adequate when switching buffers.
gand loading density, analyte concentrations, buffer conditions,
and assay step times. The data must be examined to ensure the
results are in agreement with what is known about the sample Ligand loading
and receptor, and evaluated for non-specific interactions. The amount of ligand immobilized (loaded) onto the biosensor
— whether FcRn or IgG — can have significant impact on the
Quality of reagents results of an assay in terms of signal strength, apparent kinetic
behavior, and non-specific binding. Although loading as much
Reagent quality is a critical factor with any kinetics assay.
protein as possible in the loading step will certainly maximize
Aggregation of the antibody or receptor can impact kinetics
the assay signal, this approach also has the potential to create
due to increased avidity and non-specific binding. Antibody
undesirable artifacts. Effects such as molecular crowding, avid-
samples should be fully evaluated for purity, activity and quality
ity, non-specific binding, and/or mass transport can all impact
using analytical techniques before using in a kinetics experi-
the observed binding kinetics. Conversely, if too little ligand
ment. Reagents that have been stored at 4°C for long periods,
is immobilized, assay signal may be very low, resulting in poor
especially at very high or very low concentrations, should not
separation of data traces and inadequate signal-to-noise ratio.
be used. Careful consideration should be given to purification,
Therefore ligand loading levels should be optimized for every
storage conditions and handling of receptor proteins and mul-
assay and biosensor format.
tiple freeze-thaw cycles avoided. Commercially available FcRn
can vary in quality, so determining a reliable source of high To perform a loading optimization experiment, several dilutions
quality FcRn is critical to good assay performance. FcRn used of ligand molecule are loaded in parallel onto biosensors. An
for experiments described in this application note was provided association step is performed for each ligand concentration us-
by Immunitrack. We have found this reagent to be consistent in ing the same, high concentration of analyte (10–20x the estimat-
quality and highly active. More information on FcRn from Immu- ed KD). A zero-ligand biosensor should also be run as a control
nitrack can be found at http://immunitrack.com. for determining whether the analyte binds non-specifically to
the biosensor surface. The concentration of ligand to select
Assay buffer for the optimized assay should be the lowest concentration of
immobilized ligand that yields an acceptable signal response
The strict pH dependence of the binding interaction between in the analyte association step (typically between 0.4 nm and
IgG and FcRn is fundamental for efficient recycling and rescue 0.6 nm). Figure 4 illustrates a loading optimization experiment
of IgG from intracellular degradation. For FcRn receptor kinetics for an FcRn-IgG binding assay using FAB2G biosensors. The
assays with BLI we recommend using phosphate-based assay optimal IgG loading concentration to use in this example is
buffer at pH 6.0 for dilution of analyte samples as well as base- the one that yields between 0.4–0.6 nm shift response in the
line and dissociation assay steps (100 mM Sodium Phosphate, association step, in this case around 3 μg/mL IgG. Also note
150 mM NaCl, 0.05% Tween-20, pH6.0). This assay buffer en- that the shape of the association step curves is improved at the
sures efficient binding of FcRn to IgG under acidic conditions and lower loading concentrations. The binding curves flatten out at
also minimizes non-specific binding. BSA is not recommended as equilibrium and show less heterogeneity - indicative of reduced
a buffer component, since some cross-reactivity may occur with secondary, non-specific interactions and better 1:1 kinetics.
3
Page4
A FcRn Association
IgG Loading Titration Baseline (1.6 µM) Dissociation
4.5
4.0
3.5
3.0 25 µg/mL
2.5 12.5 µg/mL
2.0 6.25 µg/mL
1.5 3.13 µg/mL
1.0 1.56 µg/mL
0.5
0 µg/mL
0
0 50 100 150 200 250 300 350 400 450 500
Time (sec)
B
FcRn association (1.6 µM) Dissociation
1.2
IgG 25 μg/mL
IgG 12.5 μg/mL
1.0 IgG 6.25 μg/mL
IgG 3.13 μg/mL
IgG .56 μg/mL
0.8
0.6
0.4
Figure 4: IgG loading optimization for FcRn assay on FAB2G biosensor.
A) Raw data traces. In the loading step, FAB2G sensor is loaded with a 2-fold di-
0.2 lution series of IgG (ligand) starting at 25 µg/mL. A zero-IgG control is also run to
assess non-specific binding of FcRn to the biosensor. In the analyte association
step, FcRn is run with each IgG concentration at a single concentration of 1.6 µM.
0 B) Overlay of FcRn association step for each IgG loading concentration.
0 20 40 60 80 110
Time (sec)
4
Binding (nm) Binding (nm)
Page5
Analyte concentration the KD when loading conditions are optimized. In this case,
choose a series of analyte concentrations that cover the range
In the association step, the rate of binding of the analyte to of the assay, for example from 20–50x KD down to the limit of
immobilized ligand is measured. Measuring a single analyte measurement. When affinity is low, e.g. in the micromolar range,
concentration can be sufficient for simple binding assays or using analyte concentration 10x above the KD may be so high as
qualitative analysis. However, when accurate kinetic and affinity to be impractical and cause non-specific binding. In this case it
constants are required, a dilution series of at least four to six is best to choose an analyte concentration range that works for
analyte concentrations must be measured in the association the particular interaction, for example starting at 2 to 5x KD and
step. Multiple analyte concentrations enable global curve titrating down to limit of measurement. As a general guideline,
fitting, where all the curves in a data set are fit simultaneously nm shift signal for the highest analyte concentration used
to yield one set of results. The analyte concentration range to should equilibrate at 0.4–0.6 nm. The lowest concentration
use will depend on the sensitivity of the assay and affinity of the signal should be higher than 0.01 nm so as to be adequately
interaction, however it should typically range from concentra- above the noise level of the instrument. Adjusting the ligand
tion of about 10x the estimated KD down to about 0.5x KD, using loading level can bring analyte signals into the optimal range
2-fold or 3-fold dilutions. (see Ligand Loading Optimization section). Figure 8 shows an
At acidic pH (pH 6–6.5), FcRn has a low micromolar to nano- example of processed data from optimized FcRn-IgG kinetic
molar affinity for the Fc region of IgG.2 When affinity is high, it assays on FAB2G biosensors.
may not be possible to see signal at concentrations at or below
2.5
2.0
1.5
Baseline FcRn
Ligand Loaded Sensors Titration
(5 µg/mL IgG) 0–1600 nM
1.0
NSB
0.5
Reference Sensors
(0 µg/mL IgG)
0
0 50 100 150 200 250 300 350 400
Time (sec)
A5 B5 C5 D5 E5 F5 G5 H5 A6 B6 C6 D6 E6 F6 G6 H6
Figure 5: Non-specific binding (NSB) of FcRn to FAB2G biosensors. Loading step for bottom (non-ligand-loaded) trace was run in 1x Kinetics buffer instead of IgG1
ligand. A high concentration of FcRn was associated to the non-ligand-loaded biosensors. NSB is indicated by positive signal in the FcRn association step for the
non-ligand-loaded biosensors, indicated by the arrow.
5
Binding (nm)
Page6
Assay step times accurate calculation of kinetic rates. In order to test for non-spe-
cific binding of analyte, run a preliminary experiment where the
FcRn-IgG interactions often have fast on-rates (>1E5 M-1s-1), concentration of ligand in the loading step is zero, and analyte
where the primary binding interaction will reach equilibrium is run at a single concentration that is well above the KD (i.e.
quickly. When the interaction is fast, the association step 10–20x estimated KD). This test can be easily included in the
should be run only for enough time for the interaction to equil- ligand loading optimization experiment described previously.
ibrate (indicated when binding traces flatten out). This time can A positive signal in the association step indicates the analyte is
be as short as 60 seconds. As long as there is curvature in the binding non-specifically to the biosensor (Figure 5).
association step data traces and equilibrium is reached for
higher analyte concentrations, a shorter association step can If the NSB signal is minimal, it can be subtracted during data
improve kinetic fitting with a 1:1 binding model. analysis by referencing (see Referencing section). However,
NSB nm shift signal that is more than 20% of maximum assay
Non-specific binding signal should be minimized by optimizing assay conditions.
Steps that can be taken to mitigate NSB include modifying the
For any biosensor kinetic assay, non-specific binding (NSB) of assay buffer and/or adding a blocking step after ligand loading.
analyte to the biosensor in the association step must be mini- Increasing the amount of Tween-20 (up to 0.05% v/v) in the
mized since it can alter analyte binding profile and interfere with buffer or increasing salt concentration can improve the strin-
Figure 6: Changing the data acquisition rate. In Octet System Data Acquisition software under the Run Experiment tab, click the pull-down menu for Acquisition
rate and select High Concentration Kinetics (10 Hz, averaging by 5). Acquisition rate should be determined based on binding rate, amount of signal generated
and experimentation.
6
Page7
gency of binding and eliminate non-specific signal. Adding a
five-minute blocking step after loading and before the kinetic
.40
baseline step using up to 0.2% casein in PBS can in some cases
be effective as well. Initial Fast Dissociation
.30
Data acquisition rate Secondary, slower
.20 dissociation indicating
stabilized binding
Because FcRn-IgG interactions often have fast binding rates, the
.10
standard rate of data acquisition (5.0 Hz, averaging by 20) in the
Octet System Data Acquisition software may not be the ideal
0
setting. When data is acquired by the Octet system, there is a 0 20 40 60 80 100 120
delay from when the biosensor dips into sample and the first data Time (sec)
points are reported to allow the software to average the collect- Figure 7: Characteristics of an optimized FcRn kinetic profile on FAB2G biosen-
ed data. This delay can cause the reported signal for the asso- sors. Processed data shown after reference sample subtraction and alignment
ciation step to initiate well above the baseline in a fast binding to baseline. Association step represents a 2-fold dilution series of FcRn, where
equilibrated signal ranges from 0.45 nm at highest concentration, down to 0.02 nm
interaction, leading to inaccuracies. If this effect is observed and for the lowest concentration. The biphasic dissociation step in FcRn-Fc binding is
impacts data fitting, the data acquisition rate can be increased to typical and may be due to dimerized FcRn molecules stabilizing the interaction.
enable more rapid reporting of binding data. The data acquisition
rate refers to the number of binding signal data points report- kinetic profile. The other conformation has two FcRn molecules
ed by the Octet system per second and is reported in Hertz. A binding a single Fc site asymmetrically as a dimer, with one
higher acquisition rate generates more data points per second FcRn molecule involved primarily in the binding interaction and
with less averaging, and monitors fast binding events better than the other contributing to stabilization of the complex. Dimeriza-
a slower acquisition rate. The rate setting can be changed in the tion is not required for binding in this model, but the presence
Advanced Settings box in the Run Experiment tab in Data Ac- of an FcRn homodimer bound to Fc increases the affinity of the
quisition software (Figure 6). Select the acquisition rate for High interaction over monomer binding. This model may explain the
Concentration Kinetics (10.0 Hz, averaging by 5). Data collected biphasic nature of the dissociation step observed in biosensor
at a higher acquisition rate may have lower signal-to-noise ratio analysis of FcRn binding to Fc. After an initial rapid dissociation
and appear noisier than data collected at standard rate. Acquisi- of singly-bound FcRn, the secondary, higher-affinity interaction
tion rate setting should always be decided based on the binding of dimerized FcRn bound to Fc begins to dominate the kinetic
rates, the amount of signal generated, as well as experimentation profile in a two-step binding interaction.
with the settings.
The 1:1 kinetic binding model can be used to fit this more com-
Data analysis plex interaction when only the first portion of the dissociation
step is included in the analysis. Truncating the dissociation step
The most accurate kinetic and affinity constants are determined fitting to 5–10 seconds enables the initial dissociation rate in
when using global data fitting method with data from several the biphasic curve to be captured so that fitting is improved
(four to six) analyte concentrations run in parallel. The Octet and reproducible off-rate and KD values can be calculated
System Data Analysis software offers several pre-programmed (Figure 8A, 8B, 8D, 8E). Using this method for curve fitting is
curve fitting models for global analysis of binding data. The most useful for ranking purposes, when FcRn binding is being
kinetic and affinity constants that are calculated depend upon compared between IgG samples or to a reference material in
the model selected. Although the solution stoichiometry of the order to determine loss or gain of activity.
interaction of FcRn with IgG Fc is 2:1, in a properly optimized
biosensor assay, the kinetic profile would be expected to follow For the purpose of reporting or publishing affinity of an interac-
a single-stoichiometric binding curve representative of a single tion displaying complexity in dissociation, the equilibrium bind-
analyte molecule per binding site – as illustrated in Figure 2B. ing constant can be calculated using Steady State analysis tools
Therefore, the 1:1 binding model is the most relevant for fitting in the Octet System Data Analysis software. The steady state
FcRn-IgG interaction with BLI. responses (where the binding trace plateaus, or equilibrates,
in the association step) for the various analyte concentrations
FcRn-IgG dissociation profiles typically display more complex are calculated using the R-equilibrium (Req) function and plotted
kinetics, however — even in an optimized assay (Figure 7). The against analyte concentration. The resulting binding isotherm
dissociation step typically appears biphasic. A possible expla- is fitted using the Langmuir model to calculate equilibrium
nation can be found in crystal structures of FcRn-Fc complex, constant KD (Figure 8C, 8F). Steady state KD is also useful for
which suggest two potential conformations for FcRn bound to ranking experiments or calculating percent activity against a
the Fc region3–5. In one, FcRn binds singly, one to each binding reference sample. Note that the kinetic KD’s using the truncated
site on opposite sides of Fc, as illustrated in Figure 2A. Binding dissociation step fitting and the steady state KD’s calculated in
in this conformation would be expected to dissociate via 1:1 the experiment in Figure 8 closely match.
7
Binding (nm)
Page8
IgG1 IgG4
A 1:1 Fitting: full dissociation D 1:1 Fitting: full dissociation
0.6 .50
.40
0.4
.30
.20
0.2
.10
0 0
0 20 40 60 80 100 120 0 20 40 60 80 100 120
Time (sec) Time (sec)
B 1:1 Fitting: partial dissociation E 1:1 Fitting: partial dissociation
0.6 .50
KD=6.77E-07 .40 KD=7.17E-07
±1.61E-08 ±2.48E-08
0.4
.30
.20
0.2
.10
0 0
0 20 40 60 80 100 120 0 20 40 60 80 100 120
Time (sec) Time (sec)
C F
Figure 8: Comparison of kinetic data fitting strategies. FAB2G biosensors were used to capture hIgG1 and hIgG4 for a binding kinetics assay with FcRn using several
concentrations (A, B, C for IgG1; D, E, F for IgG4). The 1:1 model with global fitting and 5 seconds of the dissociation step (fit lines are in red) were used to determine affin-
ity constant. KD, using the full dissociation step (A, D) and 5 seconds of the dissociation step (B, E). Steady-state analysis of data was also used to determine equilibrium
KD (C, F).
8
Binding (nm) Binding (nm)
Binding (nm) Binding (nm)
Page9
Referencing enable subtraction of non-specific binding of analyte to the
bare surface, and are considered optional in a BLI protein
Two methods of referencing are used in biosensor kinetic kinetic assay.
assays: 1) A reference sample is run on a biosensor that has
ligand present in the loading step but with zero analyte in Double referencing can be performed during data analysis,
the association step, i.e. a buffer-only negative control. When where signal from reference biosensors and reference sample
using a capture-based biosensor such as FAB2G, some are subtracted from sample data. We have found that in FcRn
background level of dissociation of the captured IgG ligand assays, subtracting out the small amount of NSB via double
from the sensor will occur. This background dissociation, or referencing generates improved kinetic profiles and data fitting
assay drift, can be subtracted out using the reference sample. over single referencing with a reference sample only. To illus-
2) Reference biosensors refer to zero-ligand biosensors that trate, Figure 10 shows the FcRn-IgG kinetic fits generated from
are dipped into buffer or irrelevant protein during the loading single vs. double referencing and respective calculated kinetic
step. Reference biosensors are run through the same analyte and affinity constants. NSB signal that is more than 20% of
samples as the ligand-loaded biosensors in a replicate assay maximum association signal should not be subtracted out, but
(Figure 9). A separate reference biosensor should be included should instead be reduced by optimizing assay conditions (see
for each analyte concentration used. Reference biosensors Non-Specific Binding section).
Biosensor tray Sample plate
1 2 3 4 5 6 7 8 9 10 11 12 1 2 3 4 5 6 7 8 9 10 11 12
A A
B B
C C
D D
E E
F F
G G
H H
Ligand biosensors Assay buer FcγR analyte (dilution series)
Reference biosensors Antibody ligand Reference sample (assay buer)
Assay Step Sensor column Step name Sample column Step type
1 1 1 Equilibration 1 Custom
1 2 1 Loading 2 Loading
1 3 1 Baseline 3 Baseline
1 4 1 Association 4 Association
1 5 1 Dissociation 3 Dissociation
2 1 2 Equilibration 1 Custom
2 2 2 Loading 1 Loading
2 3 2 Baseline 3 Baseline
2 4 2 Association 4 Association
2 5 2 Dissociation 3 Dissociation
Figure 9: Assay protocol for an experiment on FAB2G biosensors with the Octet RED96 instrument that utilizes double referencing. A reference sample is included in
the association step that contains assay buffer with no analyte to correct for baseline drift. Reference biosensors enable subtraction of non-specific binding, and are an
additional set of biosensors that are run through a replicate assay. All steps are repeated on reference biosensors except the ligand loading step, which is performed in
buffer. When Double Reference is selected in the Octet System Data Analysis software Processing window, both Reference Sample and Reference Biosensor data will
be subtracted from sample data.
9
Page10
A Single referencing B Double referencing
.7
.6
.6
.5
.5
.4
.4
.3
.3
.2
.2
.1 .1
0 0
0 20 40 60 80 100 120 0 20 40 60 80 100 120
Time (sec) Time (sec)
Single referencing Double referencing
KD 5.86E-07 6.77E-07
Figure 10: Comparison of kinetic fitting using single referencing with reference
sample vs. double referencing with both reference sample and reference bio- Kon 1.78E+05 1.83E+05
sensors. FAB2G biosensors were used to capture Herceptin (HIgG1) for a bind-
ing kinetics assay with FcRn using several concentrations. The 1:1 model with Koff 1.04E-01 1.24E-01
global fitting and 5 seconds of the dissociation step (fit lines are in red) were
used to determine affinity constant. A) Kinetic fitting using single reference. B) KD error 2.24E-08 1.61E-08
Kinetic fitting using double reference. C) Kinetics constant and fitting statistics R2 0.9972 0.9990
calculated from single reference and double reference data analysis.
χ2 0.0700 0.0226
Biosensor regeneration shows the overlay of data for association of FcRn to IgG1 immo-
bilized on FAB2G biosensors. Ten assay cycles were run, with
Regeneration of biosensors in kinetic analysis can offer savings regeneration procedure described above performed between
on biosensor costs and provide a cost-effective method for each cycle. When association/dissociation steps for each cycle
generating replicate data for ligand-analyte pairs. Efficient are overlaid, binding response does not decrease but remains
regeneration requires removing the bound analyte or ligand/ consistent with increasing number of cycles. The CVs for the
analyte complex without affecting activity of the biosensor. kinetic and affinity constants calculated for each assay cycle are
The number of regeneration cycles that can be withstood is well below 10%.
biosensor and protein dependent; some can be regenerated
ten or more cycles, while others tolerate far fewer cycles or In some cases binding capacity may decrease during the first
cannot be regenerated at all. A standard regeneration proce- regeneration cycle but stabilize for the remaining cycles. To
dure for biosensors used for studying FcRn-IgG interactions is avoid this initial change, a pre-conditioning step is recom-
exposure to 10 mM glycine pH 1.7 for five seconds followed by mended, where the regeneration protocol is performed on the
assay buffer for five seconds, repeating four times for a total of unused biosensors before beginning the assay. The number of
four exposures to regeneration buffer. Utilizing this protocol on regeneration cycles that can be performed successfully in an
FAB2G biosensors will remove IgG ligand-FcRn complex and assay will be biosensor and format-dependent, and should be
restore the original biosensor chemistry. New IgG sample can tested for each experimental system. Regeneration can also be
then be loaded for measuring another interaction. used to improve reproducibility within an assay, since it enables
multiple samples to be run on an identical biosensor surface.
If regeneration is successful, the analyte binding curves follow-
ing each regeneration cycle will overlay with minimal change in
responses when compared to earlier binding cycles. Figure 11
10
Binding (nm)
Binding (nm)
Page11
A
6
5
4
3
2
1
0
-1
-2
0 500 1000 1500 2000 2500 3000 3500 4000 4500
Time (sec)
B C
Association Dissociation Association Dissociation
.60 .60
.40 .40
.20 .20
0 0
0 20 40 60 80 100 120 0 20 40 60 80 100 120
Time (sec) Time (sec)
Figure 11: Biosensor regeneration in FcRn kinetic assays. A) Raw data: FAB2G
sensors went through pre-condition step, then 10 kinetic assay cycles with Average %CV
regeneration. Reference sensors without ligand loading were also included to KD 5.67E-07 4.1%
determine the non-specific binding of FcRn to FAB2G sensors. For each kinetic
assay, association and dissociation steps performed to a 2-fold dilution series of Kon 2.23E+05 3.5%
FcRn. B) Overlay of FcRn association/dissociation steps. C) Curve fitting of data
traces, using a 1:1 model with global fitting and a 5-second dissociation step (fit Koff 1.26E-01 2.8%
lines are in red). D) Table of average kinetic and affinity constants with CV’s for
the 10 regeneration cycles.
11
Binding (nm)
Binding (nm)
Binding (nm)
Page12
A B
KD (M) increase with H2O2 treatment
1.4E-06
H2O2 treatment (hr) KD (M)
0 5.05E-07
1.2E-06
1 6.55E-07
3 9.20E-07
5 1.32E-06 1.0E-06
8.0E-07
Figure 12: Impact of Methionine oxidation on the binding of Herceptin-FcRn y = 2E-07x + 5E-07
kinetics. H2O2 treated hIgG1 samples were analyzed for affinity to FcRn in a full R2 = 0.99007
kinetic assay. Analysis was performed using 1:1 global fitting with a portion of
the dissociation step (5 seconds). Double referencing method was used. A) 6.0E-07
Table of calculated KD’s for each oxidation time point. B) Calculated KD plotted
against time of H2O2 treatment (hr) showing trend of decreasing binding affinity
with increasing oxidation time.
4.0E-07 0 1 2 3 4 5 6
H2O2 Treatment Time (hr)
Measuring afinity of FcRn in antibody oxidation as a form of product degradation has been demon-
engineering, comparability studies, lot strated to reduce the affinity of Fc fragment to FcRn4. For this
release assays and QC experiment, oxidized antibody samples were generated via time
course incubation in 0.3% hydrogen peroxide followed by buffer
The Octet system provides a user-friendly platform that enables exchange into low pH assay buffer. The affinity of FcRn binding
rapid assay optimization and integrates readily into workflows at was measured for each of the treated hIgG1 samples as well as
many stages of antibody drug development - from early phase untreated hIgG1 control using FAB2G biosensors on the Octet
candidate selection to release of marketed product. Detailed RED384 instrument. A dilution series of FcRn was associated to
analysis of both kinetics and binding affinity are essential to un- immobilized hIgG1 samples and double referencing with 1:1 global
derstanding the activity of biotherapeutic candidates and guiding data fitting used to calculate KD and kinetic constants.
further design strategies. Equivalence of materials needs to be
established in terms of quality, safety and efficacy, including full Figure 12 shows the fitted data traces and analysis from the kinet-
comparison of immunological properties between biosimilar and a ics experiment. The results demonstrate that an increase in cal-
licensed originator product. culated KD correlates with increasing degree of oxidation of the
sample. The experiment was performed on RED384 instrument
The FcRn-IgG kinetic assays described here are well suited as an using 80 µL of sample per well in a 384-well plate. All samples
analytical tool for detailed biotherapeutic characterization as well for every time point can be run in a single walk-away assay with
as assessment of stability, comparability, and lot-to-lot consis- total running time of less than 2 hours. This experiment illustrates
tency. In order to demonstrate sensitivity of kinetic analysis on the ease with which a sensitive, high throughput characterization
the Octet system for determining changes in product quality, the assay can be established on the Octet platform for assessing
described methods of analysis were used to measure affinity of FcRn binding activity to IgG.
FcRn to stressed IgG samples compared to control. Methionine
12
KD (M)
Page13
hIgG1-FcRn hIgG4-FcRn
Intra-assay Inter-assay Intra-assay Inter-assay
Average CV% Average CV% Average CV% Average CV%
KD 5.92E-07 2.0% 5.25E-07 4.2% 6.20E-07 2.0% 6.66E-07 6.0%
kon 2.10E+05 0.6% 2.25E+05 5.1% 2.08E+05 1.7% 2.13E+05 1.4%
kdis 1.24E-01 2.5% 1.18E-01 2.5% 1.29E-01 0.4% 1.42E-01 5.0%
Figure 13: Intra-assay and Inter-assay precision of the HIgG-FcRn binding pairs.
Intra-assay and inter-assay precision system using our FAB2G biosensors, with recommendations for
assay optimization and data analysis. Careful assay develop-
To demonstrate reproducibility of FcRn kinetic assays on the ment and experimental design will consistently yield data that is
Octet platform, intra-assay and Inter-assay precision of the KDs reproducible and of high quality, making the Octet system ideal
were determined for hIgG1 and hIgG4 binding to FcRn using for cost-effective, label free kinetic analysis to complement
FAB2G biosensors. Results are shown in Figure 13. Replicate functional assays and replace more cumbersome label-free
data (n=3) were collected for each binding pair and kinetic and methods such as SPR and immunoassays.
affinity constants calculated using 1:1 binding model with trun-
cated dissociation step (Figure 13). The low percent CV values
demonstrate the high level of precision and reliability that can be References
achieved with kinetic analysis on the Octet platform when proper 1 Crystal structure and immunoglobulin G binding properties of the human ma-
assay optimization is performed. jor histocompatibility complex-related Fc receptor, West A P Jr and Bjorkman
PJ, Biochemistry, 39(32), 9698–708, 2000.
2 pH-dependent binding of immunoglobulins to intestinal cells of the neonatal
Conclusion rat, Rodewald, R, J. Cell Biol. 71, 666–669, 1976.
3 Crystal structure of the complex of rat neonatal Fc receptor with Fc, Burmeis-
Measuring accurate and reliable kinetics of interactions be- ter, W. P et al, Nature, 372, 379–383, 1994.
tween neonatal Fc receptor and monoclonal antibodies can be 4 Characterization of the 2:1 complex between the class I MHC-related Fc
challenging, but is a critical application in many stages of bio- receptor and its Fc ligand in solution, Martin W L and Bjorkman PJ, Biochem-
istry, 38:12639–47,1999.
pharmaceutical development. The Octet platform offers a rapid,
5 Crystal Structure at 2.8 Å of an FcRn/Heterodimeric Fc Complex: Mechanism
flexible, and sensitive solution for measuring these interactions, of pH-Dependent Binding, Martin, W L et al, Molecular Cell, 7, 867–877, 2001.
whether performing full kinetic analysis, steady state analysis or 6 Impact of methionine oxidation on the binding of human IgG1 to FcRn and Fc
measuring relative binding. Here we have described meth- receptors, Bertolotti-Ciarlet, A et al, Molecular Immunology, 46, 1878–1882,
ods for producing high quality FcRn kinetic data on the Octet 2009.
ForteBio ForteBio Analytics (Shanghai) Co., Ltd. Molecular Devices (UK) Ltd. Molecular Devices (Germany) GmbH
47661 Fremont Boulevard No. 88 Shang Ke Road 660-665 Eskdale Bismarckring 39
Fremont, CA 94538 Zhangjiang Hi-tech Park Winnersh Triangle 88400 Biberach an der Riss
888.OCTET-75 or 650.322.1360 Shanghai, China 201210 Wokingham, Berkshire Germany
www.fortebio.com fortebio.info@moldev.com salesops.china@moldev.com RG41 5TS, United Kingdom + 00800 665 32860
+44 118 944 8000
uk@moldev.com
©2019 Molecular Devices, LLC. All trademarks used herein are the property of Molecular Devices, LLC. Specifications subject to change without
notice. Patents: www.moleculardevices.com/product patents. FOR RESEARCH USE ONLY. NOT FOR USE IN DIAGNOSTIC PROCEDURES.
41-0241-AN Rev C